M1 protein
| Flu_M1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 influenza virus m1 protein | |||||||||
| Identifiers | |||||||||
| Symbol | Flu_M1 | ||||||||
| Pfam | PF00598 | ||||||||
| InterPro | IPR001561 | ||||||||
| SCOP2 | 1aa7 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 42 | ||||||||
| OPM protein | 1aa7 | ||||||||
| |||||||||
The M1 protein is a matrix protein of the influenza virus. It forms a coat inside the viral envelope. This is a bifunctional membrane/RNA-binding protein that mediates the encapsidation of nucleoprotein cores into the membrane envelope. It is therefore required that M1 binds both membrane and RNA simultaneously.[1]
The M1 protein binds to the viral RNA. The binding is not specific to any RNA sequence, and is performed via a peptide sequence rich in basic amino acids.[citation needed]
It also has multiple regulatory functions, performed by interaction with the components of the host cell. The mechanisms regulated include a role in the export of the viral ribonucleoproteins from the host cell nucleus, inhibition of viral transcription, and a role in the virus assembly and budding. The protein was found to undergo phosphorylation in the host cell.[citation needed]
The M1 protein forms a layer under the patches of host cell membrane that are rich with the viral hemagglutinin, neuraminidase and M2 transmembrane proteins, and facilitates budding of the mature viruses.[citation needed]
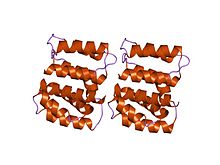
M1 consists of two domains connected by a linker sequence. The N-terminal domain has a multi-helical structure that can be divided into two subdomains.[2] The C-terminal domain also contains alpha-helical structure.
See also
Sources and notes
- ^ Sha B, Luo M (March 1997). "Structure of a bifunctional membrane-RNA binding protein, influenza virus matrix protein M1". Nat. Struct. Biol. 4 (3): 239–44. doi:10.1038/nsb0397-239. PMID 9164466. S2CID 22521750.
- ^ Arzt S, Baudin F, Barge A, Timmins P, Burmeister WP, Ruigrok RW (January 2001). "Combined results from solution studies on intact influenza virus M1 protein and from a new crystal form of its N-terminal domain show that M1 is an elongated monomer". Virology. 279 (2): 439–46. doi:10.1006/viro.2000.0727. PMID 11162800.
- v
- t
- e
| linear ds-DNA (Duplodnaviria, Varidnaviria) |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| circular ds-DNA (Duplodnaviria, Varidnaviria?) |
| ||||||||||||||||
| other (Riboviria, Monodnaviria) |
|
| ds-RNA (Riboviria) |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ss-RNA positive-sense (Riboviria) |
| ||||||||||||
| ss-RNA negative-sense (Negarnaviricota) |
| Structure and genome of HIV |
| ||||
|---|---|---|---|---|---|
| Multiple |
|











