Violdelphin
 | |
| Names | |
|---|---|
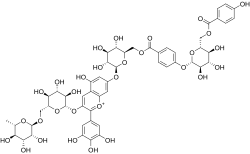
| IUPAC name 3′,4′,5,5′-Tetrahydroxy-7-(6-O-{4-[6-O-(4-hydroxybenzoyl)-β-D-glucopyranosyloxy]benzoyl}-β-D-glucopyranosyloxy)-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavylium | |
| Systematic IUPAC name (12R,13R,14R,15R,16S,42R,43S,44S,45R,46S,82S,83R,84S,85S,86R,142S,143R,144S,145S,146R)-13,14,15,43,44,45,65,83,84,85,143,144,145,184-Tetradecahydroxy-16-methyl-11,17-dioxo-62-(3,4,5-trihydroxyphenyl)-2,5,7,10,13,16-hexaoxa-61λ4-6(3,7)-[1]benzopyrana-1(2),4,8,14(2,6)-tetrakis(oxana)-12(1,4),18(1)-dibenzenaoctadecaphan-61-ylium | |
| Other names Delphinidin 3-rutinoside-7-O-(6-O-(4-(6-O-(4-hydroxybenzoyl)-beta-D-glucosyl)oxybenzoyl)-beta-D-glucoside) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C53H59O30+ |
| Molar mass | 1176.02 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Violdelphin is an anthocyanin, a plant pigment, has been found in the purplish blue flower of Aconitum chinense,[1] in the blue flowers in the genus Campanula[2] and in the blue flowers of Delphinium hybridum.[3] It is a flavenoid natural product, incorporating two p-hydroxy benzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.
References
- ^ The anthocyanin responsible for purplish blue flower colour of Aconitum chinense. Kosaku Takeda, Syuji Sato, Hiromitsu Kobayashi, Yoko Kanaitsuka, Mariko Ueno, Takeshi Kinoshita, Hiroyuki Tazaki and Takane Fujimori, Phytochemistry, June 1994, Volume 36, Issue 3, Pages 613–616, doi:10.1016/S0031-9422(00)89784-8
- ^ Structure and biosynthesis of anthocyanins in flowers of Campanula. Kirsten Brandt, Tadao Kondo, Hideki Aoki and Toshio Goto, Phytochemistry, 29 April 1993, Volume 33, Issue 1, Pages 209–212, doi:10.1016/0031-9422(93)85424-P
- ^ Structure of Violdelphin, an Anthocyanin from Violet Flower of Delphinium hybridum. Tadao Kondo, Kaori Oki, Kumi Yoshida and Toshio Goto, Chemistry Letters,1990, Vol. 19, No. 1, pages 137-138, doi:10.1246/cl.1990.137
- v
- t
- e
Anthocyanidins and their anthocyanin glucosides
- 5-Desoxy-peonidin
- Aurantinidin
- Cyanidin
- 6-Hydroxycyanidin
- Delphinidin
- Fisetinidin
- Guibourtinidin
- Pelargonidin
- Robinetinidin
- 5-Desoxy-malvidin
- Capensinidin
- Europinidin
- Hirsutidin
- Kaempferidinidin
- Malvidin
- Peonidin
- Petunidin
- Pulchellidin
- Rosinidin
(anthocyaninidin glycosides)
Glucosides:
- Callistephin (Pelargonidin 3-O-glucoside)
- Chrysanthemin (Cyanidin 3-O-glucoside)
- Myrtillin (Delphinidin 3-O-glucoside)
- Oenin (Malvidin 3-O-glucoside)
- Peonidin 3-O-glucoside
- Petunidin 3-O-glucoside
- Pulchellidin 3-glucoside
Diglucosides:
- Cyanin (Cyanidin 3,5-O-diglucoside)
- Delphin (Delphinidin 3,5-O-diglucoside)
- Malvin (Malvidin 3,5-diglucoside)
- Pelargonin (Pelargonidin 3,5-O-diglucoside)
- Peonin (Peonidin 3,5-O-diglucoside)
- Petunin (Petunidin 3,5-O-diglucoside)
Others glycosides:
- Antirrhinin (Cyanidin 3-O-rutinoside)
- Ideain (Cyanidin 3-O-galactoside)
- Delphinidin 3-O-rhamnoside
- Petunidin 3-O-arabinoside
- Petunidin 3-O-galactoside
- Petunidin 3-O-rhamnoside
- Petunidin 3-O-rutinoside
- Primulin (Malvidin 3-O-galactoside)
- Pulchellidin 3-rhamnoside
- Tulipanin (Delphinidin 3-O-rutinoside)
| Acetylated anthocyanins | |
|---|---|
| Coumaroylated anthocyanins (cis- and trans-) |
|
| Caffeoylated anthocyanins |
|
| Malonylated anthocyanins |
|
| Acylated anthocyanin diglycosides |
|
- Malvidin glucoside-ethyl-catechin
- Catechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epicatechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Afzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epiafzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Metalloanthocyanins (commelinin)
- Cyanosalvianin
- Protocyanin
- Protodelphin)
- Pyranoanthocyanins
- Copigmentation
- Anthocyanone A (degradation product of oenin)
- Malvone (oxidation product of malvin)












