Penem
A penem is a type of β-lactam with an unsaturated five-member heterocycle containing a sulfur atom in a pentacyclic ring fused to the β-lactam ring. Penems do not occur naturally; all are synthetic.[1] Related to penems are carbapenems, which have a carbon atom in place of the sulfur atom.[2]
An example is faropenem.[3]
Structure

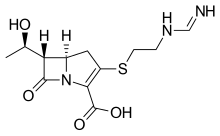

Penem molecules do not occur naturally, and production of penems is an entirely synthetic process.
Five main penem subgroups — thiopenems, oxypenems, aminopenems, alkylpenems, and arylpenems — have been produced and are distinguished by the side chain (at position 2) of the unsaturated five-membered ring. One structurally distinct penem is BRL 42715. This molecule has no substitution at the above position, but has a bulky group attached to the β-lactam ring, and it displays effective inhibition of class C β-lactamases, but no antimicrobial activity.
One possible consequence of these structural differences of penems from other β-lactams may be reduced immunogenicity and immunogenic cross-reactivity.
References
- ^ Richard Wise (1990). "The carbapenems and Penem Antibiotics—a brief review". Antimicrobic Newsletter. 7 (10): 73–78. doi:10.1016/0738-1751(90)90045-E.
- ^ "Medscape.com".
- ^ Milazzo I, Blandino G, Caccamo F, Musumeci R, Nicoletti G, Speciale A (March 2003). "Faropenem, a new oral penem: antibacterial activity against selected anaerobic and fastidious periodontal isolates". Journal of Antimicrobial Chemotherapy. 51 (3): 721–5. doi:10.1093/jac/dkg120. PMID 12615878.
Further reading
- Sasaki A, Goda K, Enomoto M, Sunagawa M (May 1992). "Synthetic studies of carbapenem and penem antibiotics. II. Synthesis of 3-acetyl-2-azetidinones by (2 + 2) cycloaddition of diketene and Schiff bases". Chemical & Pharmaceutical Bulletin. 40 (5): 1094–7. doi:10.1248/cpb.40.1094. PMID 1394625.
- v
- t
- e
(inhibit synthesis
of peptidoglycan
layer of bacterial
cell wall by binding
to and inhibiting
PBPs, a group of
D-alanyl-D-alanine
transpeptidases)
| Glycopeptides Lipoglycopeptides |
|
|---|---|
| Lipopeptides |
|
| Polymyxins |
|
| Other |
|
- Inhibit PG subunit synthesis and transport: NAM synthesis inhibition (Fosfomycin)
- DADAL/AR inhibitors (Cycloserine)
- bactoprenol inhibitors (Bacitracin)
- Hydrolyze NAM-NAG
- Tyrothricin
- Isoniazid#
- Teixobactin
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III









