Glukokortikoidni receptor
| Nuklearni receptor potfamilije 3, grupa C, član 1 (glukokortikoidni receptor) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Kristalografska struktura DNK vezujućeg domena glukokortikoidnog receptora (DBD, levo, 1R4O vezan za DNK) i ligand vezujući domen [LBD, desno, 1M2Z vezan za deksametazon (beli štapići) i TIF2 koaktivatorski protein (crveno)]. Isprekidane žute linije predstavljaju interakcije vodoničnog vezivanja između receptora i liganda. 2D struktura deksametazona je takođe prikazana u dole desno. | |||||||||||
| Dostupne strukture | |||||||||||
| 1M2Z, 1NHZ, 1P93, 3BQD, 3CLD, 3E7C, 3H52, 3K22, 3K23, 4HN5, 4HN6 | |||||||||||
| Identifikatori | |||||||||||
| Simboli | NR3C1; GCCR; GCR; GR; GRL | ||||||||||
| Vanjski ID | OMIM: 138040 MGI: 95824 HomoloGene: 30960 IUPHAR: GeneCards: NR3C1 Gene | ||||||||||
| |||||||||||
| Pregled RNK izražavanja | |||||||||||
 | |||||||||||
 | |||||||||||
 | |||||||||||
| podaci | |||||||||||
| Ortolozi | |||||||||||
| Vrsta | Čovek | Miš | |||||||||
| Entrez | 2908 | 14815 | |||||||||
| Ensembl | ENSG00000113580 | ENSMUSG00000024431 | |||||||||
| UniProt | P04150 | E9PUR6 | |||||||||
| RefSeq (mRNA) | NM_000176 | NM_008173 | |||||||||
| RefSeq (protein) | NP_000167 | NP_032199 | |||||||||
| Lokacija (UCSC) | Chr 5: 142.66 - 142.82 Mb | Chr 18: 39.41 - 39.49 Mb | |||||||||
| PubMed pretraga | [1] | [2] | |||||||||
Glukokortikoidni receptor (GR, GCR, NR3C1, nuklearni receptor potfamilije 3, grupa C, član 1) receptor je za koji se vezuju kortizol i drugi glukokortikoidi.
GR je izražen u skoro svim ćelijma a telu i reguliše gene koji kontrolišu razviće, metabolizam, i imunski respons. Gen ovog receptora se izražava u nekoliko formi, te stoga GR ima mnoštvo različitih (pleiotropskih) dejstava u različitim delovima tela.
Kad se za GR vežu glukokortikoidi, njegov primarni mehanizam dejstva je regulacija trakripcije gena.[1][2] Nevezani receptor se nalazi u ćelijskom citosolu. Nakon vezivanja glukokortikoida za receptor, receptor-glukortikoidni kompleks može da povisi izražavanje antiinflamatornih proteina u jedru ili da suzbije izražavanje proinflamatornih proteina u citozolu (putem sprečavanja translokacije drugih transkripcionih faktora iz citozola u jedro).
Kod ljudi, GR protein je kodiran NR3C1 genom koji je lociran na hromozomu 5 (5q31).[3][4]
Strukture
Poput drugih steroidnih receptora,[5] glukokortikoidni receptor ima modularu strukturu[6] i sadrži sledeće domene (obležene sa A - F):
- A/B - N-terminal regulatory domain
- C - DNK vezujući domen (DBD)
- D - region šarke
- E - ligand vezujući domen (LBD)
- F - C-terminalni domen
Vezivanje liganda i odgovor
U odsustvu hormona, glukokortikoidni receptor (GR) se nalazi u citosolu u kompleksu sa raznim proteinima uključujući protein toplotnog šoka 90 (hsp90), protein toplotnog šoka 70 (hsp70) i protein FKBP52 (FK506 vezujući protein 52).[7] Endogeni glukokortikoidni hormon kortizol difuzijom prolazi kroz ćelijsku membranu u citoplazmu i vezuje se za glukokortikoidni receptor (GR), što dovodi do oslobađanja proteina toplotnog šoka. Rezultujuća aktivirana forma GR ima dva moguća mehanizma dejstva, transaktivacija i transrepresija.[8][9]
Transaktivacija
Direktni mehanizam dejstva obuhvata homodimerizaciju receptora, translokaciju putem aktivnog transporta u jedro, i vezivanje za specifiće DNK responsivne elemente, čime se aktivira transkripcija gena. Ovaj mehanizam dejstva se naziva transaktivacija. Biološki respons zavisi od tipa ćelije.
Transrepresija
U odsustvu aktiviranog GR, drugi transkripcini faktori kao što je NF-κB ili AP-1 mogu da transaktiviraju ciljne gene.[10] Aktivirani GR može da formira kompleks as tim drugim transkripcionim faktorima i da spreči njihovo vezivanje za ciljne gene i time suzbije izražavanje gena koje normalno kontrolišu NF-κB ili AP-1. Ovaj indirektni mehanizam dejstva se naziva transrepresija.
Klinički značaj
GR je abnormalan u sučajevima familialne glukokortikoidne otpornosti.[11]
U strukturama centralnog nervnog sistema, glukokortikoidni receptor učestvuje u neuroendokrinoj integraciji. On funkcioniše kao glavna komponenta endokrinog uticaja na mozak, posebno u responsu na stres. Ovaj receptor je impliciran u kratkotrajnu i dugotrajnu adaptaciju u responsu na stresore, i važan je za razumevanje psiholoških poremećaja, uključujući pojedine tipove depresije.[12][13]
Agonisti i antagonisti
Deksametazon je agonist, a RU486 i ciproteron su antagonisti GR. Isto tako, progesteron i DHEA deluju kao antagonisti na GR.
Interacije
Glukokortikoidni receptor može da formira interakcije sa:
- BAG1,[14][15]
- CEBPB,[16]
- CREBBP,[17]
- DAP3,[18]
- DAXX,[19]
- HSP90AA1,[18][20][21][22][23][24][25]
- HNRPU,[26]
- MED1,[27][28]
- MED14,[28]
- Mineralokortikoidni receptor,[29]
- NRIP1,[27][30][31]
- NCOR1,[32][33]
- NCOA1,[27][34]
- NCOA2,[27][35]
- NCOA3,[27][36]
- POU2F1,[37][37][38]
- RANBP9,[39]
- RELA,[39][40][41]
- SMAD3,[42][43]
- SMARCD1,[36]
- SMARCA4[36][44]
- STAT3,[45][46]
- STAT5B,[47]
- Tioredoksin,[48]
- TRIM28,[49] and
- YWHAH.[50]
Reference
- ^ Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA (2006). „International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors”. Pharmacol Revl. 58 (4): 782—97. PMID 17132855. doi:10.1124/pr.58.4.9. [Free full text]
- ^ Rhen T, Cidlowski JA (2005). „Antiinflammatory action of glucocorticoids--new mechanisms for old drugs”. N. Engl. J. Med. 353 (16): 1711—23. PMID 16236742. doi:10.1056/NEJMra050541.
- ^ Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM (1985). „Primary structure and expression of a functional human glucocorticoid receptor cDNA”. Nature. 318 (6047): 635—41. PMID 2867473. doi:10.1038/318635a0.
- ^ Francke U, Foellmer BE (1989). „The glucocorticoid receptor gene is in 5q31-q32 [corrected]”. Genomics. 4 (4): 610—2. PMID 2744768. doi:10.1016/0888-7543(89)90287-5.
- ^ Kumar R, Thompson EB (1999). „The structure of the nuclear hormone receptors”. Steroids. 64 (5): 310—9. PMID 10406480. doi:10.1016/S0039-128X(99)00014-8.
- ^ Kumar R, Thompson EB (2005). „Gene regulation by the glucocorticoid receptor: structure:function relationship”. J. Steroid Biochem. Mol. Biol. 94 (5): 383—94. PMID 15876404. doi:10.1016/j.jsbmb.2004.12.046.
- ^ Pratt WB, Morishima Y, Murphy M, Harrell M (2006). „Chaperoning of glucocorticoid receptors”. Handb Exp Pharmacol. 172 (172): 111—38. PMID 16610357. doi:10.1007/3-540-29717-0_5.
- ^ Buckingham JC (2006). „Glucocorticoids: exemplars of multi-tasking”. Br J Pharmacol. 147 (Supplement 1): S258—68. PMC 1760726
 . PMID 16402112. doi:10.1038/sj.bjp.0706456.
. PMID 16402112. doi:10.1038/sj.bjp.0706456. - ^ Hayashi R, Wada H, Ito K, Adcock IM (2004). „Effects of glucocorticoids on gene transcription”. Eur J Pharmacol. 500 (1-3): 51—62. PMID 15464020. doi:10.1016/j.ejphar.2004.07.011.
- ^ Ray A, Prefontaine KE (1994). „Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor”. Proc. Natl. Acad. Sci. U.S.A. 91 (2): 752—6. PMC 43027
 . PMID 8290595. doi:10.1073/pnas.91.2.752.
. PMID 8290595. doi:10.1073/pnas.91.2.752. - ^ Mendonca B, Leite M, de Castro M, Kino T, Elias L, Bachega T, Arnhold I, Chrousos G, Latronico A (2002). „Female pseudohermaphroditism caused by a novel homozygous missense mutation of the GR gene”. J Clin Endocrinol Metab. 87 (4): 1805—9. PMID 11932321. doi:10.1210/jc.87.4.1805.
- ^ Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J (2007). „Neurobiology of depression: an integrated view of key findings”. Int J Clin Pract. 61 (12): 2030—40. PMC 2228409
 . PMID 17944926. doi:10.1111/j.1742-1241.2007.01602.x. [Free full text]
. PMID 17944926. doi:10.1111/j.1742-1241.2007.01602.x. [Free full text] - ^ Savitz J, Lucki I, Drevets WC (2009). „5HT1A receptor function in Major Depressive Disorder”. Prog Neurobiol. 88 (1): 17—31. PMC 2736801
 . PMID 19428959. doi:10.1016/j.pneurobio.2009.01.009. [Free full text]
. PMID 19428959. doi:10.1016/j.pneurobio.2009.01.009. [Free full text] - ^ Kullmann M, Schneikert J, Moll J, Heck S, Zeiner M, Gehring U, Cato AC (1998). „RAP46 is a negative regulator of glucocorticoid receptor action and hormone-induced apoptosis”. J. Biol. Chem. 273 (23): 14620—5. PMID 9603979. doi:10.1074/jbc.273.23.14620.
- ^ Schneikert J, Hübner S, Langer G, Petri T, Jäättelä M, Reed J, Cato AC (2000). „Hsp70-RAP46 interaction in downregulation of DNA binding by glucocorticoid receptor”. EMBO J. 19 (23): 6508—16. PMC 305849
 . PMID 11101523. doi:10.1093/emboj/19.23.6508.
. PMID 11101523. doi:10.1093/emboj/19.23.6508. - ^ Boruk M, Savory JG, Haché RJ (1998). „AF-2-dependent potentiation of CCAAT enhancer binding protein beta-mediated transcriptional activation by glucocorticoid receptor”. Mol. Endocrinol. 12 (11): 1749—63. PMID 9817600. doi:10.1210/me.12.11.1749.
- ^ Almlöf T, Wallberg AE, Gustafsson JA, Wright AP (1998). „Role of important hydrophobic amino acids in the interaction between the glucocorticoid receptor tau 1-core activation domain and target factors”. Biochemistry. 37 (26): 9586—94. PMID 9649342. doi:10.1021/bi973029x.
- ^ а б Hulkko SM, Wakui H, Zilliacus J (2000). „The pro-apoptotic protein death-associated protein 3 (DAP3) interacts with the glucocorticoid receptor and affects the receptor function”. Biochem. J. 349. Pt 3: 885—93. PMC 1221218
 . PMID 10903152.
. PMID 10903152. - ^ Lin DY, Lai MZ, Ann DK, Shih HM (2003). „Promyelocytic leukemia protein (PML) functions as a glucocorticoid receptor co-activator by sequestering Daxx to the PML oncogenic domains (PODs) to enhance its transactivation potential”. J. Biol. Chem. 278 (18): 15958—65. PMID 12595526. doi:10.1074/jbc.M300387200.
- ^ Jibard N, Meng X, Leclerc P, Rajkowski K, Fortin D, Schweizer-Groyer G, Catelli MG, Baulieu EE, Cadepond F (1999). „Delimitation of two regions in the 90-kDa heat shock protein (Hsp90) able to interact with the glucocorticosteroid receptor (GR)”. Exp. Cell Res. 247 (2): 461—74. PMID 10066374. doi:10.1006/excr.1998.4375.
- ^ Kanelakis KC, Shewach DS, Pratt WB (2002). „Nucleotide binding states of hsp70 and hsp90 during sequential steps in the process of glucocorticoid receptor.hsp90 heterocomplex assembly”. J. Biol. Chem. 277 (37): 33698—703. PMID 12093808. doi:10.1074/jbc.M204164200.
- ^ Hecht K, Carlstedt-Duke J, Stierna P, Gustafsson J, Brönnegârd M, Wikström AC (1997). „Evidence that the beta-isoform of the human glucocorticoid receptor does not act as a physiologically significant repressor”. J. Biol. Chem. 272 (42): 26659—64. PMID 9334248. doi:10.1074/jbc.272.42.26659.
- ^ de Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos GP (1996). „The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role”. Mol. Med. 2 (5): 597—607. PMC 2230188
 . PMID 8898375.
. PMID 8898375. - ^ van den Berg JD, Smets LA, van Rooij H (1996). „Agonist-free transformation of the glucocorticoid receptor in human B-lymphoma cells”. J. Steroid Biochem. Mol. Biol. 57 (3-4): 239—49. PMID 8645634. doi:10.1016/0960-0760(95)00271-5.
- ^ Stancato LF, Silverstein AM, Gitler C, Groner B, Pratt WB (1996). „Use of the thiol-specific derivatizing agent N-iodoacetyl-3-[125I]iodotyrosine to demonstrate conformational differences between the unbound and hsp90-bound glucocorticoid receptor hormone binding domain”. J. Biol. Chem. 271 (15): 8831—6. PMID 8621522. doi:10.1074/jbc.271.15.8831.
- ^ Eggert M, Michel J, Schneider S, Bornfleth H, Baniahmad A, Fackelmayer FO, Schmidt S, Renkawitz R (1997). „The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U”. J. Biol. Chem. 272 (45): 28471—8. PMID 9353307. doi:10.1074/jbc.272.45.28471.
- ^ а б в г д Zilliacus J, Holter E, Wakui H, Tazawa H, Treuter E, Gustafsson JA (2001). „Regulation of glucocorticoid receptor activity by 14--3-3-dependent intracellular relocalization of the corepressor RIP140”. Mol. Endocrinol. 15 (4): 501—11. PMID 11266503. doi:10.1210/me.15.4.501.
- ^ а б Hittelman AB, Burakov D, Iñiguez-Lluhí JA, Freedman LP, Garabedian MJ (1999). „Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins”. EMBO J. 18 (19): 5380—8. PMC 1171607
 . PMID 10508170. doi:10.1093/emboj/18.19.5380.
. PMID 10508170. doi:10.1093/emboj/18.19.5380. - ^ Savory JG, Préfontaine GG, Lamprecht C, Liao M, Walther RF, Lefebvre YA, Haché RJ (2001). „Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces”. Mol. Cell. Biol. 21 (3): 781—93. PMC 86670
 . PMID 11154266. doi:10.1128/MCB.21.3.781-793.2001.
. PMID 11154266. doi:10.1128/MCB.21.3.781-793.2001. - ^ Tazawa H, Osman W, Shoji Y, Treuter E, Gustafsson JA, Zilliacus J (2003). „Regulation of subnuclear localization is associated with a mechanism for nuclear receptor corepression by RIP140”. Mol. Cell. Biol. 23 (12): 4187—98. PMC 156128
 . PMID 12773562. doi:10.1128/MCB.23.12.4187-4198.2003.
. PMID 12773562. doi:10.1128/MCB.23.12.4187-4198.2003. - ^ Subramaniam N, Treuter E, Okret S (1999). „Receptor interacting protein RIP140 inhibits both positive and negative gene regulation by glucocorticoids”. J. Biol. Chem. 274 (25): 18121—7. PMID 10364267. doi:10.1074/jbc.274.25.18121.
- ^ Stevens A, Garside H, Berry A, Waters C, White A, Ray D (2003). „Dissociation of steroid receptor coactivator 1 and nuclear receptor corepressor recruitment to the human glucocorticoid receptor by modification of the ligand-receptor interface: the role of tyrosine 735”. Mol. Endocrinol. 17 (5): 845—59. PMID 12569182. doi:10.1210/me.2002-0320.
- ^ Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R (2002). „RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding”. J. Biol. Chem. 277 (29): 26238—43. PMID 12011091. doi:10.1074/jbc.M203268200.
- ^ Kucera T, Waltner-Law M, Scott DK, Prasad R, Granner DK (2002). „A point mutation of the AF2 transactivation domain of the glucocorticoid receptor disrupts its interaction with steroid receptor coactivator 1”. J. Biol. Chem. 277 (29): 26098—102. PMID 12118039. doi:10.1074/jbc.M204013200.
- ^ Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE (2002). „Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition”. Cell. 110 (1): 93—105. PMID 12151000. doi:10.1016/S0092-8674(02)00817-6.
- ^ а б в Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK (2003). „BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation”. Mol. Cell. Biol. 23 (17): 6210—20. PMC 180928
 . PMID 12917342. doi:10.1128/MCB.23.17.6210-6220.2003.
. PMID 12917342. doi:10.1128/MCB.23.17.6210-6220.2003. - ^ а б Préfontaine GG, Walther R, Giffin W, Lemieux ME, Pope L, Haché RJ (1999). „Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter”. J. Biol. Chem. UNITED STATES. 274 (38): 26713—9. ISSN 0021-9258. PMID 10480874. doi:10.1074/jbc.274.38.26713.
- ^ Préfontaine GG, Lemieux ME, Giffin W, Schild-Poulter C, Pope L, LaCasse E, Walker P, Haché RJ (1998). „Recruitment of octamer transcription factors to DNA by glucocorticoid receptor”. Mol. Cell. Biol. 18 (6): 3416—30. PMC 108923
 . PMID 9584182.
. PMID 9584182. - ^ а б Rao MA, Cheng H, Quayle AN, Nishitani H, Nelson CC, Rennie PS (2002). „RanBPM, a nuclear protein that interacts with and regulates transcriptional activity of androgen receptor and glucocorticoid receptor”. J. Biol. Chem. United States. 277 (50): 48020—7. ISSN 0021-9258. PMID 12361945. doi:10.1074/jbc.M209741200.
- ^ Nissen RM, Yamamoto KR (2000). „The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain”. Genes Dev. 14 (18): 2314—29. PMC 316928
 . PMID 10995388. doi:10.1101/gad.827900.
. PMID 10995388. doi:10.1101/gad.827900. - ^ Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT (1995). „Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids”. Mol. Endocrinol. 9 (4): 401—12. PMID 7659084. doi:10.1210/me.9.4.401.
- ^ Li G, Wang S, Gelehrter TD (2003). „Identification of glucocorticoid receptor domains involved in transrepression of transforming growth factor-beta action”. J. Biol. Chem. 278 (43): 41779—88. PMID 12902338. doi:10.1074/jbc.M305350200.
- ^ Song CZ, Tian X, Gelehrter TD (1999). „Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3”. Proc. Natl. Acad. Sci. U.S.A. 96 (21): 11776—81. PMC 18362
 . PMID 10518526. doi:10.1073/pnas.96.21.11776.
. PMID 10518526. doi:10.1073/pnas.96.21.11776. - ^ Wallberg AE, Neely KE, Hassan AH, Gustafsson JA, Workman JL, Wright AP (2000). „Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor tau1 activation domain”. Mol. Cell. Biol. 20 (6): 2004—13. PMC 110817
 . PMID 10688647. doi:10.1128/MCB.20.6.2004-2013.2000.
. PMID 10688647. doi:10.1128/MCB.20.6.2004-2013.2000. - ^ Lerner L, Henriksen MA, Zhang X, Darnell JE (2003). „STAT3-dependent enhanceosome assembly and disassembly: synergy with GR for full transcriptional increase of the alpha 2-macroglobulin gene”. Genes Dev. 17 (20): 2564—77. PMC 218150
 . PMID 14522952. doi:10.1101/gad.1135003.
. PMID 14522952. doi:10.1101/gad.1135003. - ^ Zhang Z, Jones S, Hagood JS, Fuentes NL, Fuller GM (1997). „STAT3 acts as a co-activator of glucocorticoid receptor signaling”. J. Biol. Chem. 272 (49): 30607—10. PMID 9388192. doi:10.1074/jbc.272.49.30607.
- ^ Stöcklin E, Wissler M, Gouilleux F, Groner B (1996). „Functional interactions between Stat5 and the glucocorticoid receptor”. Nature. 383 (6602): 726—8. PMID 8878484. doi:10.1038/383726a0.
- ^ Makino Y, Yoshikawa N, Okamoto K, Hirota K, Yodoi J, Makino I, Tanaka H (1999). „Direct association with thioredoxin allows redox regulation of glucocorticoid receptor function”. J. Biol. Chem. 274 (5): 3182—8. PMID 9915858. doi:10.1074/jbc.274.5.3182.
- ^ Chang CJ, Chen YL, Lee SC (1998). „Coactivator TIF1beta interacts with transcription factor C/EBPbeta and glucocorticoid receptor to induce alpha1-acid glycoprotein gene expression”. Mol. Cell. Biol. 18 (10): 5880—7. PMC 109174
 . PMID 9742105.
. PMID 9742105. - ^ Wakui H, Wright AP, Gustafsson J, Zilliacus J (1997). „Interaction of the ligand-activated glucocorticoid receptor with the 14-3-3 eta protein”. J. Biol. Chem. 272 (13): 8153—6. PMID 9079630. doi:10.1074/jbc.272.13.8153.
Literatura
- Adcock IM, Ito K (2000). „Molecular mechanisms of corticosteroid actions.”. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace / Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Università di Napoli, Secondo ateneo. 55 (3): 256—66. PMID 10948677.
- Chikanza IC (2002). „Mechanisms of corticosteroid resistance in rheumatoid arthritis: a putative role for the corticosteroid receptor beta isoform.”. Ann. N. Y. Acad. Sci. 966: 39—48. PMID 12114257. doi:10.1111/j.1749-6632.2002.tb04200.x.
- Neeck G, Kluter A, Dotzlaw H, Eggert M (2002). „Involvement of the glucocorticoid receptor in the pathogenesis of rheumatoid arthritis.”. Ann. N. Y. Acad. Sci. 966 (1): 491—5. PMID 12114309. doi:10.1111/j.1749-6632.2002.tb04252.x.
- Yudt MR, Cidlowski JA (2003). „The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene.”. Mol. Endocrinol. 16 (8): 1719—26. PMID 12145329. doi:10.1210/me.2002-0106.
- Torrego A, Pujols L, Picado C (2003). „[Response to glucocorticoid treatment in asthma. The role of alpha and beta isoforms of the glucocorticoid receptor]”. Arch. Bronconeumol. 38 (9): 436—40. PMID 12237016.
- Bray PJ, Cotton RG (2003). „Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms.”. Hum. Mutat. 21 (6): 557—68. PMID 12754700. doi:10.1002/humu.10213.
- Kino T, Pavlakis GN (2004). „Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1.”. DNA Cell Biol. 23 (4): 193—205. PMID 15142377. doi:10.1089/104454904773819789.
- Lu NZ, Cidlowski JA (2004). „The origin and functions of multiple human glucocorticoid receptor isoforms.”. Ann. N. Y. Acad. Sci. 1024 (1): 102—23. PMID 15265776. doi:10.1196/annals.1321.008.
- Kino T, Chrousos GP (2004). „Human immunodeficiency virus type-1 accessory protein Vpr: a causative agent of the AIDS-related insulin resistance/lipodystrophy syndrome?”. Ann. N. Y. Acad. Sci. 1024 (1): 153—67. PMID 15265780. doi:10.1196/annals.1321.013.
- Andersen JL, Planelles V (2005). „The role of Vpr in HIV-1 pathogenesis.”. Curr. HIV Res. 3 (1): 43—51. PMID 15638722. doi:10.2174/1570162052772988.
- Le Rouzic E, Benichou S (2006). „The Vpr protein from HIV-1: distinct roles along the viral life cycle.”. Retrovirology. 2 (1): 11. PMC 554975
 . PMID 15725353. doi:10.1186/1742-4690-2-11.
. PMID 15725353. doi:10.1186/1742-4690-2-11. - Muthumani K; Choo AY; Premkumar A; et al. (2006). „Human immunodeficiency virus type 1 (HIV-1) Vpr-regulated cell death: insights into mechanism.”. Cell Death Differ. 12 (Suppl 1): 962—70. PMID 15832179. doi:10.1038/sj.cdd.4401583.
- Zhou J, Cidlowski JA (2005). „The human glucocorticoid receptor: one gene, multiple proteins and diverse responses.”. Steroids. 70 (5-7): 407—17. PMID 15862824. doi:10.1016/j.steroids.2005.02.006.
- Chrousos GP, Kino T (2006). „Intracellular glucocorticoid signaling: a formerly simple system turns stochastic.”. Sci. STKE. 2005 (304): pe48. PMID 16204701. doi:10.1126/stke.3042005pe48.
- Plotkin LL; Labutin AL; Lebedev LV; et al. (1975). „[Balloon probe for the removal of emboli and thrombi]”. Meditsinskaia tekhnika (3): 42—3. PMID 1152650.
- Subramaniam M; Colvard D; Keeting PE; et al. (1993). „Glucocorticoid regulation of alkaline phosphatase, osteocalcin, and proto-oncogenes in normal human osteoblast-like cells.”. J. Cell. Biochem. 50 (4): 411—24. PMID 1469072. doi:10.1002/jcb.240500410.
- Scherrer LC, Pratt WB (1992). „Association of the transformed glucocorticoid receptor with a cytoskeletal protein complex.”. J. Steroid Biochem. Mol. Biol. 41 (3-8): 719—21. PMID 1562545. doi:10.1016/0960-0760(92)90411-B.
- Cadepond F; Gasc JM; Delahaye F; et al. (1992). „Hormonal regulation of the nuclear localization signals of the human glucocorticosteroid receptor.”. Exp. Cell Res. 201 (1): 99—108. PMID 1612132. doi:10.1016/0014-4827(92)90352-9.
- Hurley DM; Accili D; Stratakis CA; et al. (1991). „Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance.”. J. Clin. Invest. 87 (2): 680—6. PMC 296359
 . PMID 1704018. doi:10.1172/JCI115046.
. PMID 1704018. doi:10.1172/JCI115046. - Encío IJ, Detera-Wadleigh SD (1991). „The genomic structure of the human glucocorticoid receptor.”. J. Biol. Chem. 266 (11): 7182—8. PMID 1707881.
Vidi još
- Selektivni agonist glukokortikoidnog receptora (SEGRA)
Spoljašnje veze
- Human Protein Reference Database
- Glucocorticoid+receptors на US National Library of Medicine Medical Subject Headings (MeSH)
- FactorBook GR
- п
- р
- у
-
 1gdc: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora
1gdc: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora -
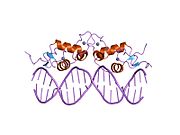 1glu: Kristalografska analiza interakcija glikokortikoidnog receptora sa DNK
1glu: Kristalografska analiza interakcija glikokortikoidnog receptora sa DNK -
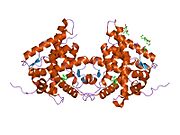 1m2z: Kristalna struktura dimernog kompleksa ljudskog ligan vezujućeg domena glukokortikoidnog receptor vezanog za deksametazon i TIF2 koaktivatorski motiv
1m2z: Kristalna struktura dimernog kompleksa ljudskog ligan vezujućeg domena glukokortikoidnog receptor vezanog za deksametazon i TIF2 koaktivatorski motiv -
 1nhz: Kristalna struktura antagonistne forme glukokortikoidnog receptora
1nhz: Kristalna struktura antagonistne forme glukokortikoidnog receptora -
 1p93: Kristalna struktura agonistne forme glikokortikoidnog receptora
1p93: Kristalna struktura agonistne forme glikokortikoidnog receptora -
 1r4o: Kristalografska analiza interakcije glukokortikoidnog receptora sa DNK
1r4o: Kristalografska analiza interakcije glukokortikoidnog receptora sa DNK -
 1r4r: Kristalografska analiza interakcije glukokortikoidnog receptora sa DNK
1r4r: Kristalografska analiza interakcije glukokortikoidnog receptora sa DNK -
 1rgd: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora iz NMR podataka putem proračuna relaksacione matrice
1rgd: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora iz NMR podataka putem proračuna relaksacione matrice -
 2gda: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora
2gda: Refinirano rešenje strukture DNK vezujućeg domena glikokortikoidnog receptora


















